What is teeth whitening?
And how does the teeth whitening process work?
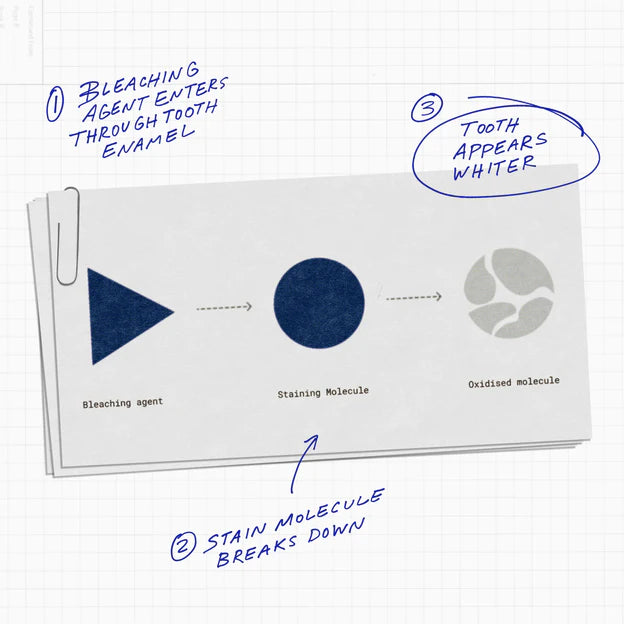
Tooth discoloration and staining result from the adhesion of various dyes and pigments to the organic matter of the tooth. The removal of these stains is typically achieved through two methods: physical removal and chemical bleaching.
Physical bleaching works on surface stains (daily surface stains) using an abrasive method. Chemical bleaching works on both surface and embedded stains (stains embedded in the tooth enamel), making it the most common and effective method of teeth whitening.

So how does chemical whitening remove stains from teeth?
Teeth whitening through chemical bleaching oxidizes stains and alters the molecules responsible for maintaining color. Simply put, the bleaching agents penetrate the tooth enamel and break these molecules down into smaller, simpler forms. The smaller the molecule, the lighter the color, resulting in an overall whiter appearance of the teeth.
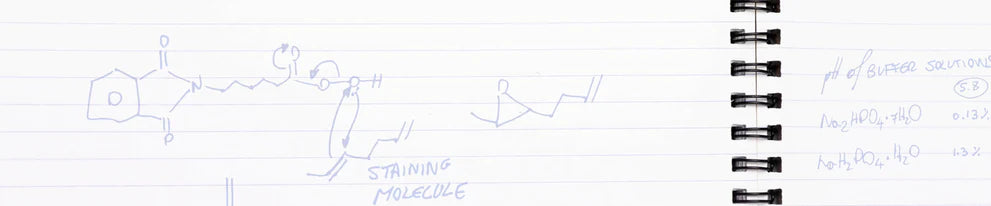
Some common bleaching agents are hydrogen peroxide, carbamide peroxide, and phthalimidoperoxycaproic acid (we call it PAP+ for short).
What is the difference between peroxide and PAP+?
Both PAP+ whitening and peroxide-based teeth whitening treatments use oxidizing action to remove stains and brighten the appearance of teeth.
As part of this process, peroxide releases something known as free radicals. Free radicals readily attack organic molecules to reduce discoloration, but they can also cause unwanted side effects such as sensitivity, gum irritation, and demineralization.
PAP+ reacts similarly to tooth stains, but without releasing free radicals. This means that the molecules responsible for the discoloration are safely broken down, without risk of sensitivity, pain, or damage.

Why isn't PAP+ used more?
Most dentists still use peroxide-based whitening treatments in their practices, as professional application in the office is more likely to limit the side effects of free radical oxidation. However, with the increasing availability and popularity of at-home peroxide products, the safety of these treatments is becoming questionable at best.
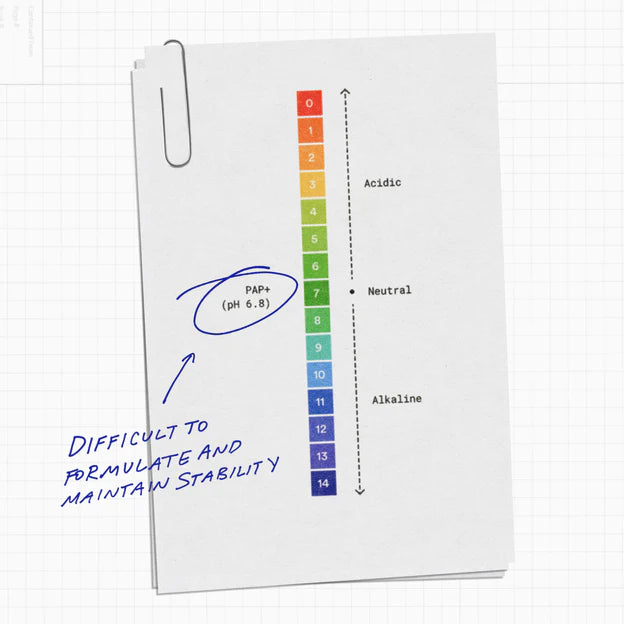
The use of PAP+ has not been widely explored by dentists, possibly due to its complex formulation and stability. The higher the pH level of PAP+, the greater its effectiveness. Unfortunately, this also means that the formula loses its efficacy after a short storage period.
What makes PAP+ special?
We have dedicated years to perfecting the optimal formulation process that maximizes the benefits of PAP+ while maintaining its stability. The result is PAP+, a formula that effectively whitens teeth without any unwanted side effects.
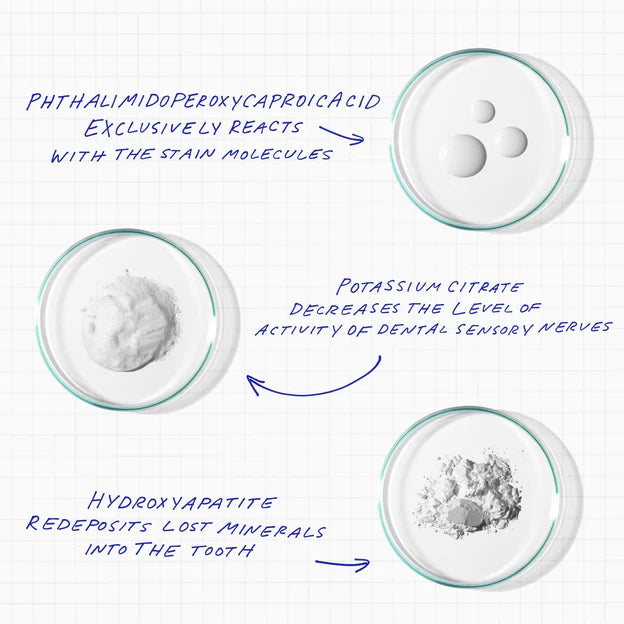
We have also added other ingredients to PAP+ that remineralize teeth and combat the effects of pre-existing sensitivity, such as potassium citrate and hydroxyapatite.